Abstract
The immunoglobulin superfamily (IgSF) is a large, diverse family of proteins extensively targeted for treatment of cancers and autoimmune diseases. Most of the therapeutic strategies targeting this family have focused on high affinity antibodies binding a single receptor. Wild-type IgSF receptors typically exhibit low affinities for their counter-structures, limiting their utility in therapeutic modulation of immune responses. We have developed a novel variant Ig domain™ (vIgD™) directed evolution platform to affinity mature human IgSF extracellular domains. In this platform, libraries of mutagenized IgSF domains are selected for altered affinity to specific recombinant protein counterstructures. Fc fusion proteins incorporating the resulting engineered IgSF domains are then tested in vitro for their ability to either agonize or antagonize T cell responses.
We successfully engineered the IgSF protein ICOSL, which binds to the costimulatory molecule ICOS on T cells and triggers T cell activation. We used our directed evolution platform to engineer a first-in-class single vIgD domain with higher affinity for ICOS and high affinity binding to the costimulatory molecule CD28, which ICOSL does not normally bind. Following the creation of dual ICOS/CD28 antagonism, the resulting domains were used to generate individual constructs encoding Fc-fusion proteins. These enhanced ICOS/CD28 dual antagonist Fc-fusion proteins were transfected into HEK-293 cells and protein produced by these cells was purified by protein A affinity chromatography. The resultant purified proteins were then tested in a FACS based binding assay on cells transfected with each costimulatory receptor showing up to a 2-3-fold increase in ICOS binding and high affinity binding to CD28. Various ICOS/CD28 dual antagonist Fc-fusion proteins were then tested in soluble format over a range of concentrations for functional activity in mixed lymphocyte reaction cultures (MLR) where allogeneic dendritic cells (DC) were used to stimulate purified human T cells. Most Variants showed significant ability to inhibit the MLR response as read out measuring proliferation and cytokine production. The potency of the Fc-fusion proteins varied somewhat depending on their relative affinities for CD28 and ICOS, with all being superior to wild type ICOSL protein and many showing superiority to the CD28 blocking reagent belatacept (CTLA4-Fc). The level of inhibition correlated well with binding of the Fc-fusion proteins to the T cells in culture.
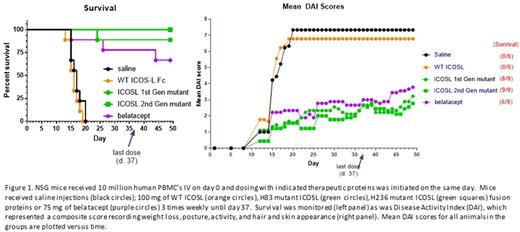
To assess the function of the ICOS/CD28 dual antagonist Fc-fusion proteins in vivo, a subset were tested in the human PBMC-NSG™ GvHD mouse model. This model monitors graft versus host disease generated by xenogeneic responses of T cells in human PBMCs transferred into NSG™. Administration of the dual ICOS/CD28 antagonist Fc-fusion proteins significantly protected mice from the effects of xenogeneic T cell activation in vivo with treated animals showing dramatically enhanced survival and greatly reduced disease scores. Interestingly, the level of protection correlated with the potency of the molecules in the in vitro MLR assay with less potent molecules in MLR assays failing to protect while more potent molecules protected well. Belatacept was also effective in protecting animals from GvHD, but not as well as the ICOS/CD28 dual antagonists produced by our vIgD platform. Collectively, these data demonstrate the vIgD directed evolution platform can generate IgSF proteins with altered affinities and improved binding properties. Variants engineered for desired immunological properties can effectively perturb T cell costimulation and affect T cell responses both in vitro and in vivo with the in vitro potency of the proteins correlating to in vivo potency. The dual ICOS/CD28 antagonists produced with the vIgD platform can be used therapeutically in disease settings where attenuation of T cell responses is beneficial, including GvHD and potentially other autoimmune diseases. Phase I enabling studies are in progress.
Levin: Alpine Immune Sciences: Employment, Equity Ownership. Evans: Alpine Immune Sciences: Employment, Equity Ownership. Rickel: Alpine Immune Sciences: Employment, Equity Ownership. Wolfson: Alpine Immune Sciences: Employment, Equity Ownership. Dillon: Alpine Immune Sciences: Employment, Equity Ownership. Kornacker: Alpine Immune Sciences: Employment, Equity Ownership, Patents & Royalties. Swanson: Alpine Immune Sciences: Employment, Equity Ownership, Patents & Royalties. Peng: Alpine Immune Sciences: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal